LATEST NEWS

From the lathe to retirement – Kalevi’s 45 years at Lojer
Kalevi Lähdekorpi built an impressive career at Lojer over nearly 46 years. During that time, he witnessed from a front-row seat the development of workshop work and the remarkable...
Lue lisää
Roberto Quintero Named CEO
Leadership Change at Lojer: Roberto Quintero Named CEO Starting 1 September, Roberto Quintero will assume the role of CEO of Lojer Oy and its subsidiary, Merivaara Oy.
Lue lisää
Pihla Salonen and Lojer Continue Their Collaboration – Aiming for the Olympics
Finnish judo rising star Pihla Salonen and Lojer continue their fruitful collaboration. The journey toward the Olympics is filled with hard work, passion, and a strong support netw...
Lue lisää
Merivaara Participates in Presidential Delegation to Kenya and Tanzania
Merivaara had the honour of joining the official business delegation accompanying the President of Finland, Alexander Stubb and his wife Suzanne Innes-Stubb, on their first state v...
Lue lisää
Merivaara Introduces New 4K Camera for Q-Flow Surgical Lights
Merivaara's latest innovation, the new Q-Flow 4K wireless camera for surgical lights, enhances surgical images with unmatched clarity and seamless integration. Unlike external came...
Lue lisää
Leadership Changes at Merivaara AB
We are pleased to welcome Johan Wallentin as our new Sales Director/Commercial Leader at Merivaara AB. Johan joined us on February 3rd, bringing with him extensive experience from ...
Lue lisää
Meet us on Arab Health 2025 in Dubai
Arab Health is one of the world's largest and most important healthcare trade fairs, held annually in Dubai, United Arab Emirates. It is an important platform for showcasing health...
Lue lisää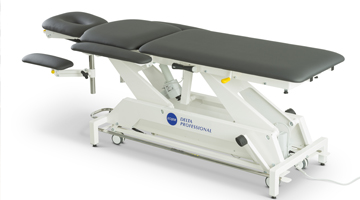
Lojer Unveils Updated Delta Series Treatment Tables
We are excited to announce the launch of updated Lojer Delta series of treatment tables, a cornerstone of our commitment to quality and innovation in healthcare.
Lue lisää
Lojer Manuthera 242 – now available in a stunning carbon color!
Solve spine-related problems with the redesigned, carbon-colored Manuthera 242 – the ultimate tool for therapists who specialize in back care.
Lue lisää
Finnish design victorious in international Red Dot competition
Merivaara is thrilled to announce that its new medical lights, Merivaara Q-Flow 1 and Q-Flow 2, have been honored with the prestigious Red Dot Product Design Award for 2024.
Lue lisää
Kinos X treatment table awarded with honorable mention at Plootu Fennica sheet metal product design competition
Plootu Fennica awards yearly products and components that sheet metal has had the essential role in the innovativeness, structure, manufacturing, or in-design of the product or com...
Lue lisää
New sales company for Lojer in Kazakhstan
Lojer's strong growth continues with the opening of a new sales company, Lojer Medical LLP, in Kazakhstan in spring 2024. Maryana Almenova has been appointed as a director of the n...
Lue lisää
 Acute and
Acute and Delivery Unit
Delivery Unit Hospital
Hospital Nursing
Nursing Homecare
Homecare Outpatients
Outpatients Physio-
Physio- Surgery and
Surgery and Veterinary
Veterinary